Weinberg College scientists reveal structure of key chromatin-remodeling complex
Breakthrough could have implications for treating a wide variety of human cancers
By Rebecca Lindell
Northwestern University researchers have mapped a group of proteins that play a critical role in both gene expression and repairing damaged DNA. By understanding this protein complex, called SWI/SNF, researchers hope to better understand how cancer arises.
SWI/SNF regulates the structure of chromatin, which comprises genetic material in a cell’s nucleus and often mutates as cancer develops.
“Mutations of this essential complex have been found in more than 20 percent of all human cancers associated with a wide range of tissue types,” said Northwestern’s Yuan He, who led the study. “Understanding the molecular mechanism of the SWI/SNF complex in regulating chromatin structure and gene transcription is thereby essential for a complete understanding of how chromatin structure alterations lead to cancer.”
The paper was published March 11 in the journal Nature. Yuan He is an assistant professor of molecular biosciences in Northwestern’s Weinberg College of Arts and Sciences and a member of the University's Robert H. Lurie Comprehensive Cancer Center.
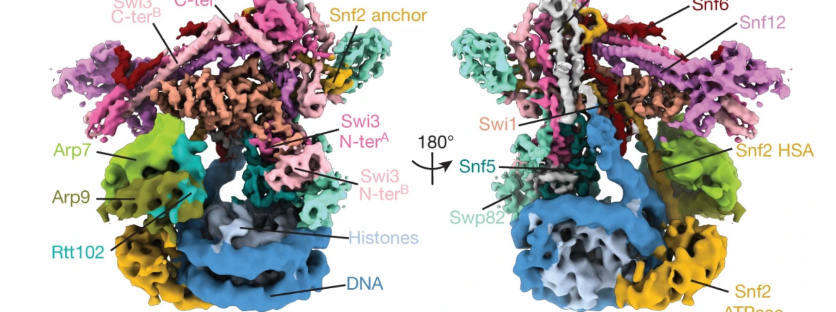
Using cryo-EM
To determine the unique structure, the He lab used cryogenic electron microscopy (cryo-EM), a powerful technique capable of revealing the 3D shape of large protein complexes. The technique involves flash-freezing proteins at a speed where water molecules don’t have time to re-organize to form crystalline ice. These protein complexes are then directly imaged by an electron microscope, and their 3D shape can then be reconstructed in 3D using a supercomputing cluster. Before cryo-EM, researchers mainly used X-ray crystallography, which is incapable of capturing high-resolution images of important complexes such as this.
This landmark study is the first time that researchers have used cryo-EM to determine the SWI/SNF structure bound to a nucleosome, at near-atomic resolution. “cryo-EM is a revolutionary technique,” said Carole LaBonne, chair of the Department of Molecular Biosciences and co-leader of the Lurie Cancer Center’s Tumor Environment and Metastasis Research Program.
“It is taking over in the critical field of structural biology. Every major research university is making major investments in this field because it is clear that it holds the key to unraveling many unanswered questions in biomedical science.”
Potential for new therapeutics
Professor Yuan He explained that the structure will allow researchers to map and rationalize cancer-related mutations in the human SWI/SNF complex.
He added that the study will provide the molecular platform for better understanding the important functions of SWI/SNF in both healthy and cancerous states, as well as for developing potential therapeutic strategies for human malignancies.
“Our study gives insight into how the complex suppresses tumor development,” He said. “And it could contribute to developing therapeutics for cancers harboring mutations in genes encoding the SWI/SNF complex.”
The study, “Cryo-EM structure of SWI/SNF complex bound to a nucleosome,” was supported by a Cornew Innovation Award from the Chemistry of Life Processes Institute at Northwestern University, the American Cancer Society (award number IRG-15-173-21) and the National Institutes of Health (award number R01GM135651, P01CA092584, U54CA193419 and 5T32 GM008382).
Back to top